October 21, 2025
 Joint hypermobility syndrome (JHS) and hypermobile Ehlers–Danlos syndrome (hEDS) fall under the umbrella of hypermobility spectrum disorders (HSDs), a range of complex multisystem connective tissue disorders that affect joint stability. Unstable joints are associated with debilitating limitations in overall mobility due to reduced biotensegrity – the elastic tension generated by myofascial tissues that governs movement quality and load transfer efficiency during physical activity.
Joint hypermobility syndrome (JHS) and hypermobile Ehlers–Danlos syndrome (hEDS) fall under the umbrella of hypermobility spectrum disorders (HSDs), a range of complex multisystem connective tissue disorders that affect joint stability. Unstable joints are associated with debilitating limitations in overall mobility due to reduced biotensegrity – the elastic tension generated by myofascial tissues that governs movement quality and load transfer efficiency during physical activity.
In the past, joint hypermobility was attributed mostly to ligament laxity, where the tissues connecting bone to bone are longer and looser than average, diminishing the support they provide. But new research is shifting focus to the role of fascia and its impact on joint stability. Learn about HSDs, how they are diagnosed, and the most recent holistic approaches to improve biotensegrity and joint stability in JHS/hEDS patients.
Joint Hypermobility Syndrome (JHS) and Hypermobile Ehlers-Danlos Syndrome (hEDS) share certain similar characteristics, but they are distinctly different conditions that require specific treatment approaches. Ehlers-Danlos syndrome is caused by genetic mutations that affect collagen proteins, and has more rigid diagnostic criteria. There are 13 subtypes of EDS, with hEDS being the most common. EDS is characterized by joint hypermobility, skin hyperextensibility, and tissue fragility, while JHS can range from asymptomatic hypermobility to symptomatic cases that fall short of meeting EDS criteria.
Characteristics of JHS include:
Unlike JHS, hypermobile Ehlers-Danlos syndrome (hEDS) is a hereditary connective tissue disorder that primarily affects collagen structure and function. Collagenous tissues include ligaments, tendons and fascia, all of which have limited vascularity, making them slow to heal from trauma and overuse.
Characteristics of hEDS include:
Traditionally, joint hypermobility disorders, particularly hypermobile Ehlers-Danlos syndrome (hEDS) and hypermobility spectrum disorders (HSD), have been considered rare, with a prevalence of about 1:5000. However, recent research is challenging that statistic, citing overly-rigid diagnostic criteria that exclude a broad spectrum of patients with symptomatic joint hypermobility.
According to the Ehlers-Danlos Society, prevalence of hEDS varies widely from one subtype to another, and in 2017 the organization created a diagnostic checklist that has been broadly used as the gold standard. But critics insist that the criteria are much too strict, causing thousands of patients to be undiagnosed or misdiagnosed with other conditions such as fibromyalgia, chronic fatigue syndrome, or other connective tissue disorders.

Hypermobility spectrum disorder (HSD) is more common than hEDS, with some estimates suggesting that 10-20% of the general population could be affected by some degree of joint hypermobility, despite not meeting the conventional diagnostic criteria. The prevalence of HSD diminishes with age, as connective tissues naturally lose some of their elastic properties and become stiffer.
When assessed with tools like the Beighton score, generalized joint hypermobility is evident in 10-30% of people, predominantly affecting children, females, and certain ethnic groups. Some athletes, especially dancers, gymnasts, and martial artists, tend to have a higher prevalence of joint hypermobility, but much of that is trained and not genetic. Prevalence of Ehlers-Danlos syndrome is rarer than generalized HSD, ranging from 1:20,000 to 1:200,000, depending on the subtype.
Conventional diagnosis of JHS is mostly joint-focused and symptoms-based, while hypermobile EDS (hEDS) often involves skin, organs, and other tissues. In both JHS and hEDS, initial diagnostic criteria are based on the Beighton Score — a diagnostic tool for joint hypermobility that assesses joint flexibility in 5 areas of the body, with a maximum score of 9 – the higher your score, the greater your joint hypermobility.
You can use the Beighton Score to assess your own joint mobility:
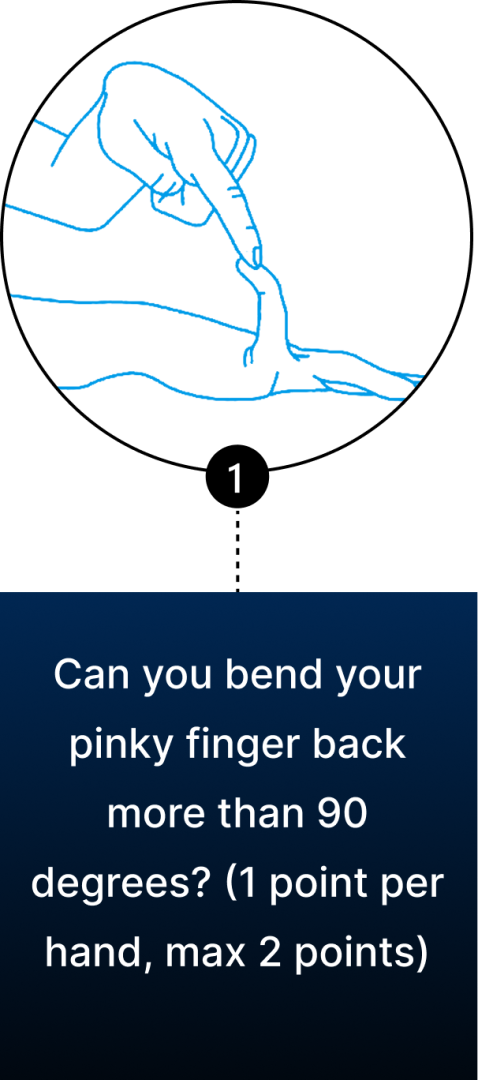
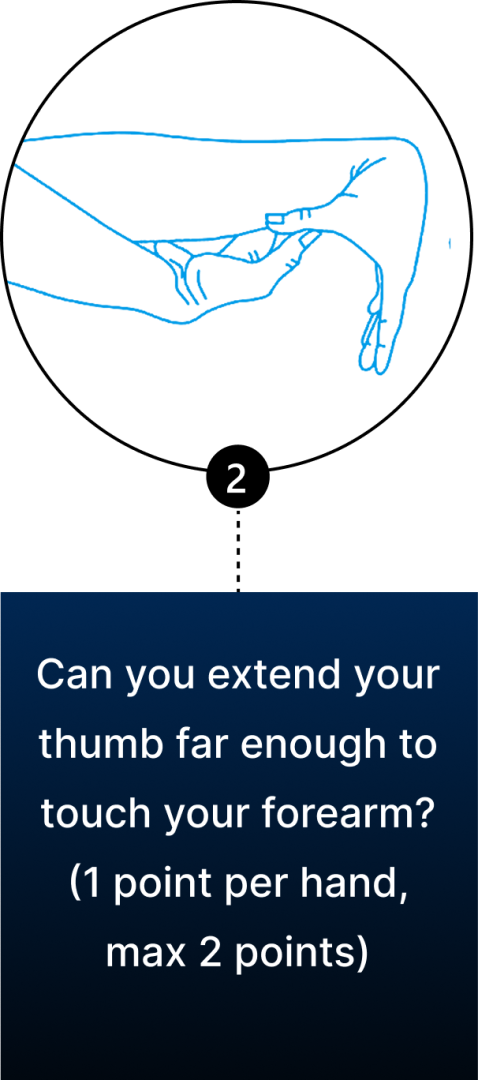
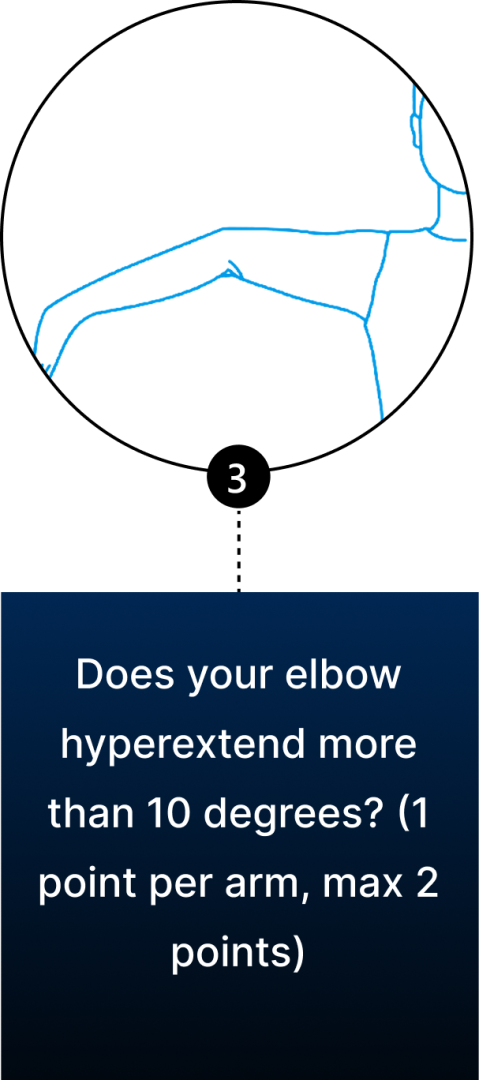
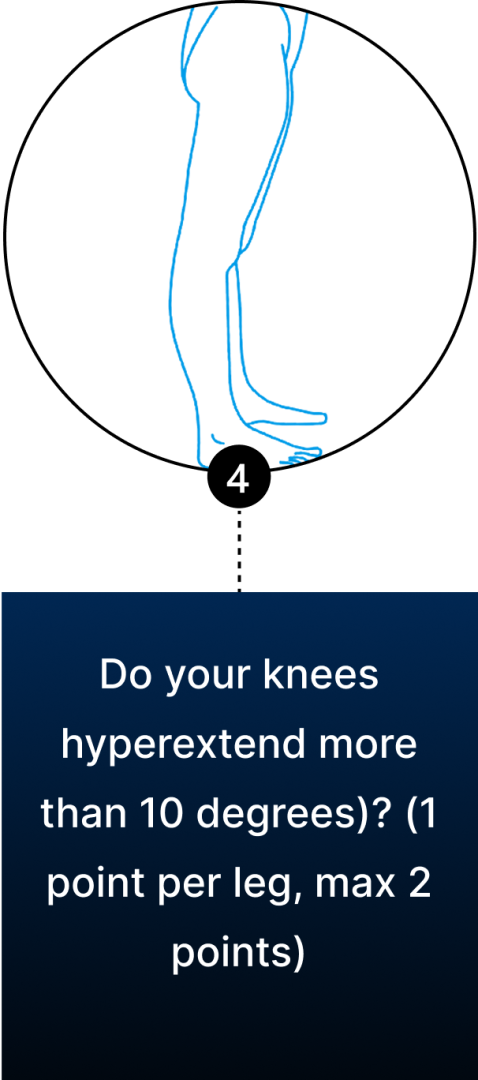
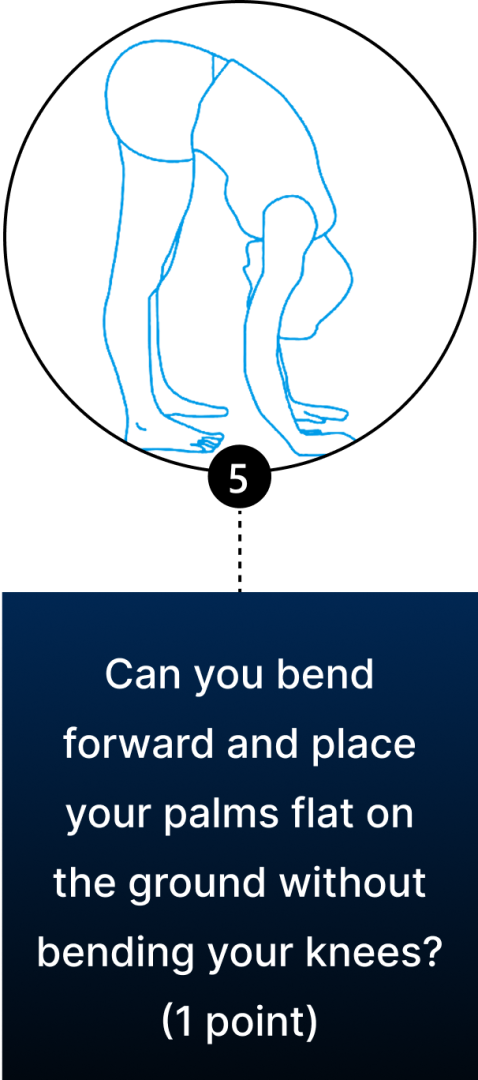
Clinicians typically use the Beighton Score in conjunction with other diagnostic criteria, such as reported symptoms of skin hyperextensibility, tissue fragility, chronic pain, and family history
Diagnosis of hEDS is subject to the Ehlers-Danlos Society’s 2017 hEDS criteria, divided into three parts:
While Ehlers-Danlos syndrome has a genetic component, there is no specific diagnostic genetic marker for hEDS. The clinician’s expertise and experience play a key role in obtaining an accurate diagnosis. Over-reliance on standardized guidelines can lead to misdiagnosis or underdiagnosis, resulting in inappropriate treatment that can sometimes make the condition worse.
Additional diagnostic tools can provide valuable information about the patient’s unique condition:
Fascia is a dynamic, fibrous web of connective tissue, densely embedded with proprioceptors, that separates, connects and encases muscles, joints, organs, nerves and blood vessels throughout the body. Fascial tissue is tough, elastic and slippery, allowing it to stretch and glide among the body’s structures without friction. Fascia works together with muscles to guide and control movement, and to provide tensile joint support via biotensegrity – elastic tension that holds structures in place as you move.

Fascia is dynamic and adaptable, but when injured, inflamed or dehydrated, it can become dense and sticky, losing its functional properties and causing pain and instability. Until recently, joint hypermobility was mostly attributed to lax ligaments – the collagenous connective tissues that connect bone to bone at the joints. But new research is turning the spotlight on fascia and its role in joint mobility and stability.
A new 2025 review of studies details the various ways that fascial dysfunction is linked to joint hypermobility. The authors found evidence of fascial thickening, reduced interfascial gliding, tendon elongation, reduced tissue stiffness, and activation of myofibroblasts (associated with the development of fibrosis) – all characteristics that appear in JHS/hEDS patients.
Fascial dysfunction can affect JHS/hEDS patients in multiple ways:

In normal patients, densifications spread along specific fascial lines that connect coordination centers throughout the body, along unique but predefined anatomical pathways. In JHS/hEDS patients, such pathways are difficult to identify and treat due to loss of biotensegrity that suppresses sensations of referred pain.
Even after myofascial release therapy, fascial densifications can re-form unless action is taken to re-establish motor control and retrain the stabilizing muscles. If left untreated, fascial densifications can turn into fibrotic tissues, reducing joint range of motion and giving the appearance of improved stability. But the appearance of stability can be misleading, because abnormal range of motion places additional stress on ligaments, leading to microinstabilities, and causing the brain to reroute force loads to more mobile joints, which in turn creates systemic imbalances that disrupt efficient mechanics.
Treatment of joint hypermobility begins with addressing fascial densifications and improving the stabilizing action of muscles. The best way to effect fascial changes in hypermobility patients is via Stecco fascial manipulation, an evidence-based technique developed by Dr. Carla Stecco, a world-renowned leader in fascial research.
The Stecco technique of fascial manipulation is a highly systematic approach that requires specific training. Unlike other manual myofascial therapies, when applied correctly, Stecco Fascial Manipulation not only restores tissue gliding – it reactivates sensory receptors within the fascia, recalibrating the neuromuscular system and restoring efficient motor control. The Stecco technique also influences the neurohumeral, paracrine, hormonal, and visceral-somatic systems, supporting whole body regulation and recovery.
The Stecco method should be administered by a certified and experienced practitioner. Attempts at fascial release by unqualified practitioners can worsen hypermobility symptoms by removing protective densifications that support joint stability, subjecting the patient to a vicious cycle of increased instability, potential trauma, and chronic pain.
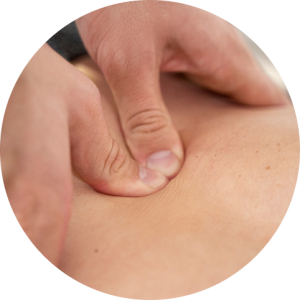
Once densifications are removed and muscle stabilization is improved, a series of Prolotherapy injections can help to tighten the ligaments surrounding hypermobile joints. Once ligaments are tightened, densifications are likely to re-form along more established fascial connections, improving force transmission and enhancing biotensegrity.
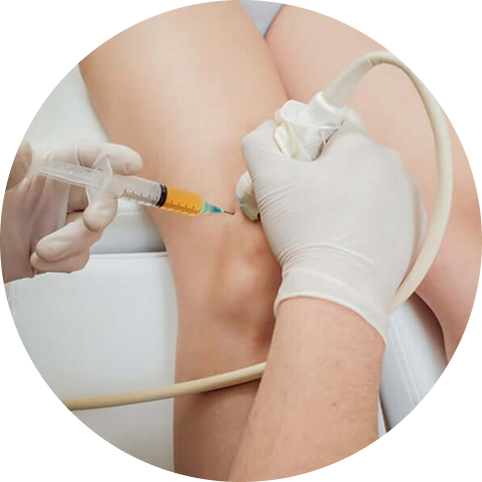
It is important to note that, once fascial tissues become fibrotic, they cannot be released manually. In such cases, a multifaceted approach involving hydrodilation injections and shockwave therapy, followed by Prolotherapy injections to ligaments, can help restore tissue integrity.
Joint hypermobility can be confounding for practitioners with minimal experience in hypermobility spectrum disorders. In many cases, well-meaning but misguided treatment can actually worsen symptoms of pain and instability, dramatically impacting the patient”s quality of life. At the same time, overly-rigid diagnostic criteria can exempt some patients from accurate diagnosis, leading to undertreatment or mistreatment that fails to produce beneficial results.
At NYDNRehab, we supplement standardized diagnostic criteria with high-resolution diagnostic ultrasound, to dynamically visualize the body’s structural interactions in real time. Dynamic imaging lets us detect abnormal muscle firing patterns and restrictions caused by densified fascia. Sonoelastography lets us test for tissue stiffness, to identify areas where biotensegrity has been compromised.
If you or a loved one struggles with hypermobile joints, contact NYDNRehab today, and get the treatment you need to improve joint stability and restore efficient movement, so you can enjoy a better and more active quality of life.
26.12 (2025): 5587
https://www.mdpi.com/1422-0067/26/12/5587
Dr. Lev Kalika is a world-recognized expert in musculoskeletal medicine. with 20+ years of clinical experience in diagnostic musculoskeletal ultrasonography, rehabilitative sports medicine and conservative orthopedics. In addition to operating his clinical practice in Manhattan, he regularly publishes peer-reviewed research on ultrasound-guided therapies and procedures. He serves as a peer reviewer for Springer Nature.
Dr. Kalika is an esteemed member of multiple professional organizations, including: